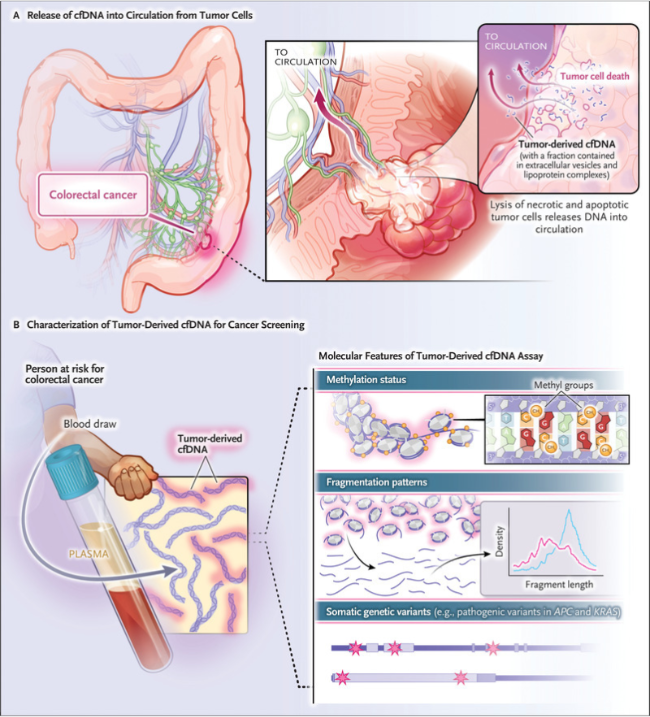
he Colorectal cancer It has a high prevalence around the world. Efforts are increasing for early detection and treatment of the disease. In this sense, Chung and colleagues from Harvard Medical School (Boston, USA) used a non-invasive test for the virus. free DNA (ctDNA) In plasma to detect disease in an average-risk population in the United States.
Free DNA is found in body fluids, such as plasma and urine. They can be analyzed using methods sensitive enough to detect low concentrations, such as PCR screening and sequencing. the DNA methylation It is the most common epigenetic signature analyzed. To know the methylation status of a fragment, it can be treated with a reagent that cleaves or binds to it, depending on whether it is methylated or not. This was the approach used by Chung's team. A more recent development is the direct detection of methylation by… Single molecule sequencing From ctDNA.
Tumor-derived DNA can be found in plasma-free DNA obtained from cancer patients, a finding that supports the experimental use of this molecule for disease detection, monitoring, and diagnosis.
The research led by Chung used a commercially available test to analyze ctDNA in the plasma of 7,861 people with an average age of 60. The test recognizes and then combines three types of information about a person's DNA: methylation status, aberrant fragmentation patterns, and the presence or absence of somatic disease-causing variants in Genes Armored personnel carrier And Brochure (Figure 1). Using this test, the sensitivity of detecting colorectal cancer was 83% Advanced tumors 90% and Advanced precancerous lesions (Included Adenomatous polyps advanced and Sessile toothed pests) by 13%. The specificity of detecting advanced tumors was inversely associated with age.
Figure 1: Detection of colorectal cancer using free DNA (ctDNA).
Although in this work they demonstrated the feasibility of using plasma ctDNA to detect disease, the relatively low sensitivity in the case of advanced precancerous lesions represents a limitation. Moreover, colonoscopy not only detects these lesions with high sensitivity, but also allows them to be removed immediately. However, the non-invasive nature of plasma ctDNA screening is an advantage that will likely lead to greater acceptance of… Colonoscopy, for which it is necessary to evaluate the cost-benefit and thus justify its implementation. Stool tests are also an option, as reported in other studies.
Although the trial of Chung et al used different information on ctDNA, the relative contributions of each of these components to the final outcome are weak and may vary between patients and populations. The possibility that test specificity decreases with age, which is thought to be related to DNA methylation, also requires further investigation. The fact that false positives can occur in people with cancers other than colorectal represents another line of study. Indeed, aberrant ctDNA methylation and fragmentation have been reported for multiple tumor types.
According to the authors of the work, the test manufacturer recommends an interval of 3 years between this type Molecular tests. A plasma DNA CT study to detect nasopharyngeal cancer has shown that people with a positive test, but without an immediately identifiable tumor, have a higher risk of developing the disease in the future. If a similar phenomenon occurs in people at risk for colorectal cancer, perhaps the interval for repeat ctDNA screening could be adapted or, conversely, conventional invasive screening could be scheduled according to the results of the previous ctDNA test.
Bibliographic source
Cell-free DNA for colorectal cancer screening
Y.M. Denis Lo, P.M., P.C.H., De Ville, D.M
State Key Laboratory of Translational Oncology, Department of Chemical Pathology
N Engl J Med 2024; 390:1047-1050

“Creator. Devoted pop culture specialist. Certified web fanatic. Unapologetic coffee lover.”